An Australian-wide survey has found approximately 1 in 5 Australian adults know a friend or family member who has experienced ongoing side effects from a COVID-19 vaccine dose they have had.
Brisbane-based Murphy’s Law Accident Lawyers surveyed 1,035 Australians aged 18+ via Google Surveys. The results showed 19.6% of survey participants answered ‘Yes’ to ‘Are you aware of any friends or family who have suffered suspected ongoing adverse side effects from a COVID-19 vaccine?’
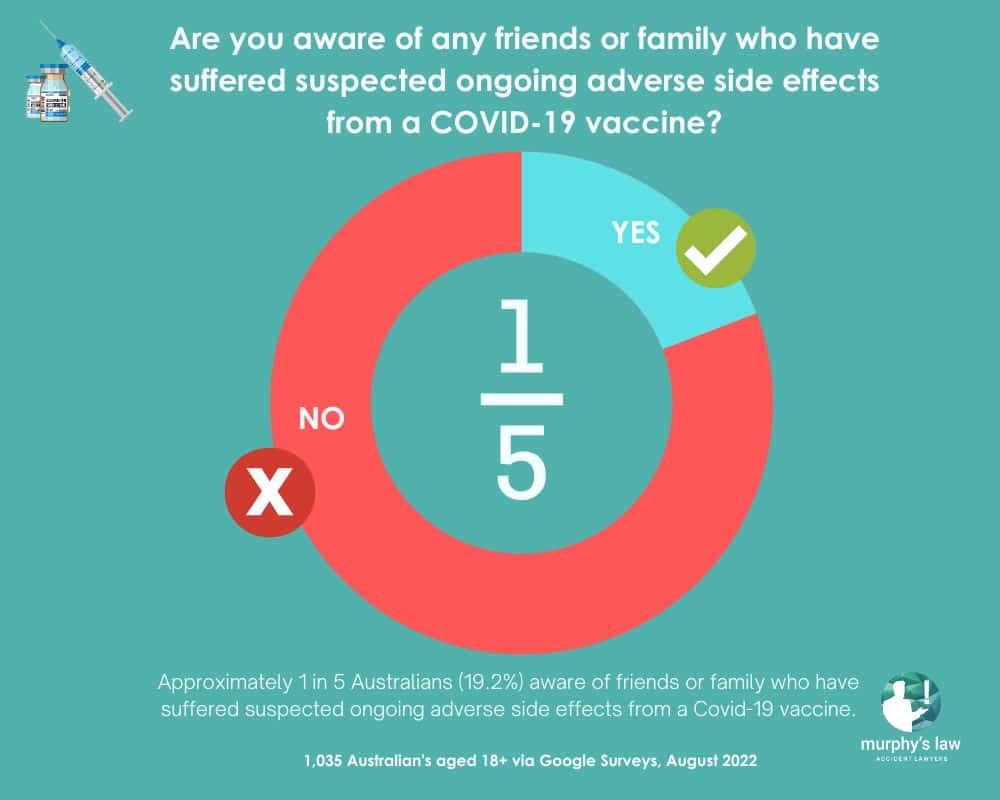
Survey results also showed those aged 18-34 were 1.5 times more likely (26.5%) to know friends and family members with suspected ongoing side effects than those aged 35+ (16.8%).
The results were fairly similar across Australian states and territories, although, as shown in the table below, the percentage of respondents aware of friends and family with ongoing side effects in Victoria, at 21.1%, is notably higher than Queensland at 16.3%.
Table: Percentage of survey respondents aware of friends or family who suffered suspected ongoing adverse side effects from a COVID-19 vaccine
| Australia Wide | Queensland | NSW | Victoria |
| 19.6% | 16.3% | 18% | 21.1% |
Donna McManus, founder of Murphy’s Law Accident Lawyers, said about the findings, “This survey polled more than 1,000 participants of all ages from right across Australia in relation to the COVID-19 vaccines collectively. The fact that almost 20% of surveyed participants were aware of at least one friend or family member who they believe has suffered not only a vaccine side-effect, but an ongoing vaccine side-effect, should be enormously concerning for everyone.’
The Effects Of The COVID-19 Vaccine
Since the Australian COVID-19 vaccine roll out began in February 2021, over 63 million doses have been administered across the country. Studies which have found vaccines can be effective in reducing severe illness with Covid-19 in the months following vaccination have been widely reported. But the side-effects, which can have debilitating outcomes and appear to be far more commonly experienced in the community (as this survey demonstrates) receives much less attention.
To date, over 135,000 adverse event reports from the COVID-19 vaccine have been lodged with the Therapeutic Goods Administration (TGA). Most of these were for mild or short-term effects after the vaccine was administered, but rarer and more severe side effects included myocarditis (inflammation of the heart muscle) and blood clots with thrombosis (which can cause further injuries, such as a stroke).
The Vaccine Claims Scheme
To help ensure the success of the vaccine rollout, the Australian government last year announced the COVID-19 Vaccine Claims Scheme. This scheme allows eligible claimants to access compensation for a series of recognised moderate to severe vaccine side effects.
While the scheme conditions might seem relatively straightforward, there are many uncertainties that are making these claims complex for claimants, including evidentiary requirements and uncertain criteria.
Many people who have sought help so far with Murphy’s Law Accident Lawyers have experienced difficulty with accessing this scheme, particularly, with the requirement that their doctor must ‘advise the specific harm suffered was most likely caused by the COVID-19 vaccine and that other circumstances are less likely’.
Discussing this issue, Kirk Watterston, Senior Associate at Murphy’s Law Accident Lawyers, stated that, ;‘A large portion of the medical fraternity are unwilling to commit their opinions to paper that there is a link between the vaccine and the injuries being sustained. Many seem happy to tell the injured parties that they believe the two are linked but are reluctant to write that in a letter, referral, or report.’
Solicitor David Lewis noted further potential issues, ‘Some people seem to be developing conditions that are listed as side effects from the manufacturer but not listed under the scheme, as well as some that aren’t identified by either. Additionally, people have had an eligible condition but have suffered follow on effects from that, for example thrombosis and/or myocarditis from AstraZeneca leading to a stroke, which in turn has led to partial blindness. The consequences and follow-on effects from these eligible conditions are not currently covered under the scheme or recognised by the manufacturer.’
What if You Suspect You Have Suffered Vaccine Side Effects?
- Seek medical assistance: If you consult a medical professional who is not receptive to the idea that the condition is a vaccine side effect, you will need to continue searching until you locate a doctor who is open-minded and willing to consider this possibility. Only then can thorough testing occur to determine the source of the side effect/or the treatment options.
- Report the side effects: You should also report the side effects to the appropriate departments, such as the TGA, to ensure your results are included in adverse event data. Reporting your side effects will give the Therapeutic Goods Administration (TGA) an accurate record of side effects and may provide data to help expand the scheme in the future.
What if You Believe Your Side Effects Are Not Covered by the Scheme?
If you believe you’ve suffered a severe side effect with ongoing impacts then you should:
- Ensure you’ve reported the injury
- Keep getting medical help to minimise the injury and impacts
- Contact Murphy’s Law and ask to be added to our ‘Vaccine Injury Register’ so you can be notified if your conditions become eligible under changes to the scheme or future class actions
Donna McManus said, ‘To be fair and just, the scheme needs significant changes to broaden its effect and to give clarification and guidance to claimants. In defiance of the evidence, some injuries are recognised as scheme injuries after receipt of some vaccines but not others. The scheme is also restricted to only the most widely-publicised injuries while other debilitating injuries are arbitrarily excluded.’
As new evidence and research emerges, it’s possible the scheme will be updated. If you do know a friend or family member suffering from ongoing side effects, then suggest they check if they are eligible under the government scheme and stay updated in case things change.
